Members of the U.S. pharmaceutical industry have been preparing their systems and business processes to meet Drug Supply Chain Security Act (DSCSA) requirements.*
Many in the U.S. pharmaceutical industry have chosen to use Electronic Product Code Information Services (EPCIS) to support their DSCSA data exchange implementation, which enables trading partners to share information about the physical movement and status of products.**
To support their work, industry approached GS1 US about establishing a program to evaluate EPCIS messages for their conformance to the GS1 US Implementation Guideline: Applying GS1 Standards for DSCSA and Traceability.
The goal is to support and streamline trading partner on-boarding with a mechanism to provide a level of confidence about readiness, implementation quality and consistency.
GS1 US Rx EPCIS Conformance Testing Program
Industry Need for Rx EPCIS Conformance Testing
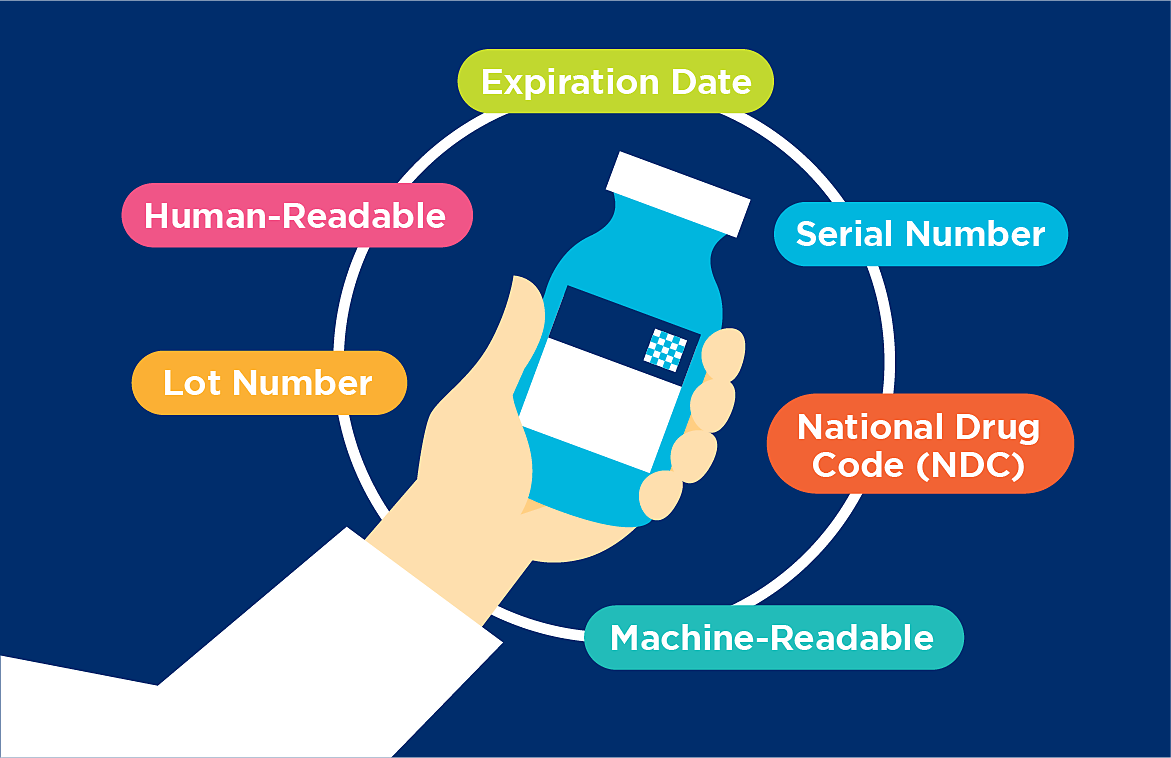
EPCIS is a GS1 Standard that enables trading partners to share information about the physical movement and status of products. It helps answer the "what, where, when, and why" questions for accurate and detailed product information.
The Program – What is it?
The GS1 US Rx EPCIS Conformance Testing Program is a voluntary program offered to support pharmaceutical industry members implementing EPCIS for DSCSA and traceability pursuant to the GS1 US Implementation Guideline. The Program is designed to validate that an EPCIS event file follows the format and structure defined in the GS1 US Implementation Guideline to support interoperable EPCIS baseline functionality for the purposes of serialized item data exchanges. It is designed for the sender of EPCIS events (whether the company\themselves, or a solution provider on their behalf), also known as “EPCIS event generators.” (Note: the program is not designed for receivers of events.)
- Pharmaceutical supply chain members who will exchange EPCIS events pursuant to the GS1 US Implementation Guideline
- Companies that will generate EPCIS events pursuant to the GS1 US Implementation Guideline
- Solution Providers (on behalf of a manufacturer, wholesaler or distributor)
GS1 US will certify third-party testing services to administer the conformance tests (“certified conformance testing service”). GS1 US will oversee program and award GS1 US Conformance Trustmarks to EPCIS event generators for each role-based test scenario they pass.
GS1 US Rx EPCIS Testing Service Certification Program Pricing Structure
- All GS1 US Rx EPCIS Testing Service Certification Program companies must be members in good standing in the GS1 US Solution Partner Program.
- The GS1 US Rx EPCIS Testing Service Certification Program has an initial certification fee of $5,000.
- If a re-test is required, the re-testing fee will be $2,000.
Certified Third-Party Conformance Testing Services
Conformance testing under this Program will be conducted by third-party testing services certified by GS1 US (“certified conformance testing service”). Certified conformance testing services will be able to conduct conformance testing for all test scenarios. GS1 US will certify third-party testing services that satisfy the Certification Requirements for Third-Party Testing Services defined in the GS1 US Rx EPCIS Conformance Testing Program Guide.
GS1 US provides this certification as a convenience and this does not constitute or imply an endorsement, recommendation or favoring by GS1 US of any identified companies, products or services. GS1 US does not warrant or guarantee any of the products or services identified, nor does it assume any legal liability or responsibility with respect to them.
Additional Resources
- Program Guide: GS1 US Rx EPCIS Conformance Testing Program PDF (1.55 MB)
- Test Scenarios: GS1 US EPCIS Rx Conformance Test Scenarios, Phase 1 and 2 XLS (20.6 KB)
- Test Cases: GS1 US EPCIS Rx Compliance Test Plan Service Requirements XLS (33.7 KB)
- GS1 US Implementation Guideline: Applying GS1 Standards for DSCSA and Traceability
- EPCIS Version 1.2 and Core Business Vocabulary (CBV) Version 1.2
- Assessing Current Implementation of DSCSA Serialization Requirements
- GS1 Standards for DSCSA for Suppliers Online Certificate Course
- White Paper - GS1 Standards Use in Clinical Research Supply Chain PDF (642 KB)
*For information about the act, see the 2013 Drug Supply Chain Security Act
**For more information, see the U.S. FDA Guidance for Industry: DSCSA Standards for the Interoperable Exchange of Information for Tracing of Certain Human, Finished, Prescription Drugs: How to Exchange Product Tracing Information (November 2014)
Disclaimer: GS1 US is the local GS1 Member Organization that supports implementation of the GS1 System in the United States. GS1 US employees are not representatives or agents of the U.S. FDA, and the content herein has not been reviewed, approved, or authorized by the U.S. FDA.


